Comparison of Roto-lined PE with FRP Bisphenol Epoxy in Ship Scrubber Piping Applications
This paper reviews the Chemical corrosion resistance of HDPE roto-lined pipes with that of Bisphenol Epoxy resin based FRP Piping in Ship Scrubbers for SOX removal from marine engine exhaust gases. Analysis of Industrial research data presented in this paper shows that roto-lined HDPE has superior corrosion resistance in Ship scrubber applications compared to Bisphenol Epoxy resin based FRP.
Background
Sulfur and Nitrogen Oxides pollution abatement is continuing to be as critical as ever in the context of tighter environmental emission norms and increasing concerns of global warming. The Process chemistry and unit operations associated with SOX and NOX removal techniques are standard and mature. One of the major challenges associated with the technologies is the selection of appropriate materials of construction that provide the lowest total cost of ownership and a high level of corrosion resistance. Exotic metallic alloys and FRP have been the popular materials of construction (MOC) for SOX Scrubbing systems in Ships primarily due to lack of better MOC. In recent years, the advent of newer materials and fabrication techniques have increased the material options available like roto-lined PE. This paper focuses on the relative advantages of roto-lined Polyethylene (PE) Pipes over Bisphenol epoxy FRP material.
Scrubber system – Chemistry and Corrosive Conditions
SOx are formed by a Ship’s Engines during the combustion process to the oxidation of naturally occurring sulphur in the fuel. In the exhaust gas, the major part of SOx is present as SO2, and a minor portion is SO3.
Reactions involving SO2:
Gaseous SO2 dissolves in seawater and subsequently is ionized, generating bisulphite and sulphite ions.
The generated hydrogen ions, are neutralized by the alkalinity of seawater (mainly due to its bicarbonate content), thereby consuming alkalinity. Reactions involving SO3 :
Gaseous SO3 dissolves in water, forming sulphuric acid, which dissociates to sulphate.

The generated hydrogen ions are neutralized by the alkalinity of seawater (mainly due to its bicarbonate content), thereby consuming alkalinity.(1)
In regions, where alkalinity of Seawater is not adequate and where no effluent discharge is permitted, a closed loop scrubbing process with normal water and external alkali dosing can be employed. The Arctic, Northwest Pacific and Norwegian ocean are some of the areas where alkalinity of Seawater is inadequate for open loop scrubbing. In the interest of brevity, a detailed description of the closed loop process is not included in this paper. The corrosive conditions encountered in the open loop process is more aggressive than that in the open loop process since sea water is more corrosive than normal water and hence the open loop process covered in this paper may be considered as a worst-case scenario.
The surface pH of Seawater usually ranges from 8.1 to 8.9. During scrubbing, the hydrogen ions generated are neutralized by the alkalinity of the Seawater resulting in a pH of 4 to 5. The corresponding concentration of Sulphuric acid can be read off the chart in Fig. 2 which gives the Sulphuric acid concentration against corresponding pH values.
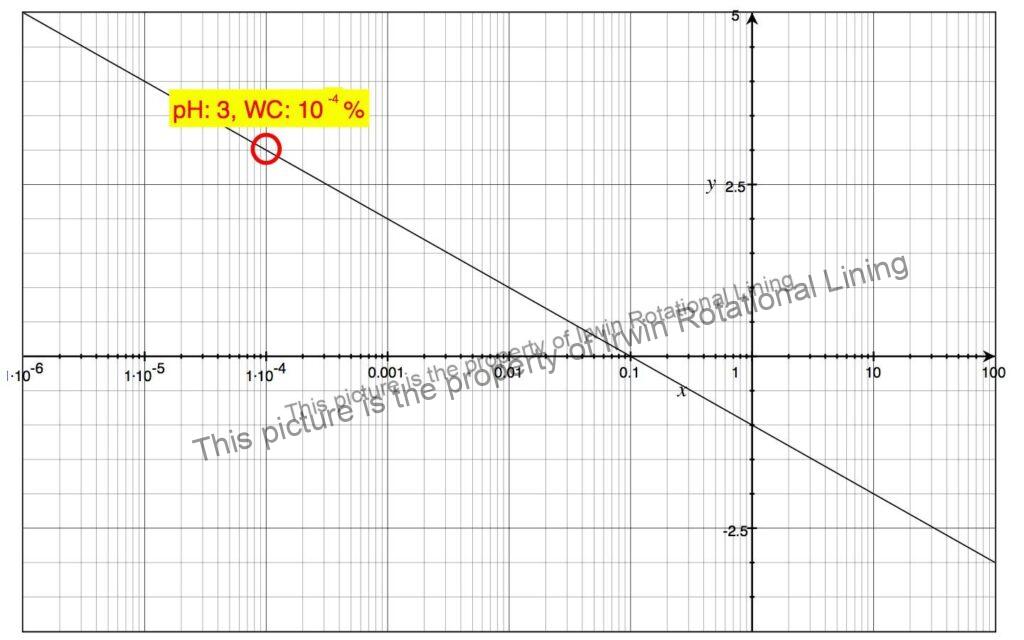
Fig 1. Sulphuric Acid Concentration vs Solution pH (2)
Since the concentrations of Sulfuric acid encountered in Ship engine exhaust gas scrubbing systems are in the dilute range as shown above, materials used in these services need to be resistant to dilute sulfuric acid and water.
Absorption of Water & other Chemical Media by Bisphenol Epoxy FRP & Polyethylene (PE)
Water absorption is a very critical parameter that impacts several properties of BE-FRP & PE materials. The properties impacted can range from corrosion resistance , Glass Transition temperature ( Tg ), strength, modulus, creep behaviour and service life. FRP consists of a resin matrix reinforced by Glass Fibre. The corrosion resistance of FRP materials are dependent on the integrity of the resin fibre-matrix bond. Water can diffuse along the fibre–matrix interface and adversely affect the fibre–matrix bond leading to loss of integrity of the fibre–matrix interface region (3). Generally, the higher the temperature of the environment and the longer the exposure time is, the larger the decrease in strength and modulus of GRP will be. A reduction in the mechanical properties by water absorption has been attributed to the debonding of the fibre–matrix interface, which leads to delamination and cracking combined with plasticizing of the matrix.
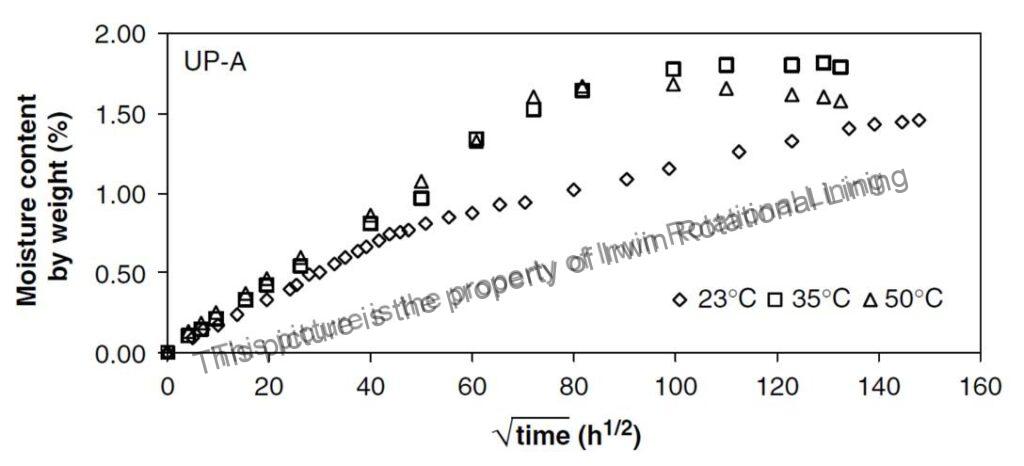
Fig. 2 Water absorption kinetics of UP-A (Unsaturated polyvinyl Ester FRP) mechanical specimens (4)
Fig.2 shows the Water absorption kinetics of an Unsaturated polyvinyl ester FRP specimen at various temperatures.
Polyethylene is very resistant to water and water vapour and has one of the lowest water absorption values of all commercial plastics. Table 1 lists the moisture absorption by LDPE & HDPE, both of which have moisture absorption values lower than 0.01%. According to Quenos (5), the absorption of water after immersion for one year at room temperature is only 0.15% by weight when measured on a disc 1 mm thick and 53 mm in diameter. This is very low compared to the moisture absorption of ~ > 1% for the same time period by Unsaturated polyvinyl Ester FRP as shown in Fig.2.Water absorption kinetics data for HDPE is not readily available in literature, probably since it is very low and does not pose an issue in most applications.
Table 1 Moisture absorption by common Plastic materials (6)
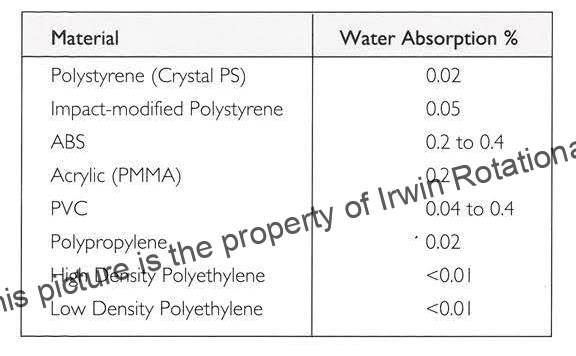
This significant difference in water absorption between Polyester / Vinyl ester FRP and LDPE/HDPE is a strong motivator for users to look at LDPE/HDPE as a potential replacement for FRP.
In the earlier section of this article on the Sulphuric acid concentration encountered in Ship engine exhaust gas scrubbing systems, it was indicated that the concentration of sulphuric acid is in very dilute range ( less than 0.1%) . Fig.3 shows the absorption of dilute Sulphuric acid (of concentration ~ 0.5% by weight) of less than 0.02% at 40 C in HDPE which is lower than the water absorption values of ~ 0.8% for Unsaturated Polyester-vinyl Ester FRP) the same value of Square root of immersion time (t1/2) shown in Fig.2. We have chosen to make this comparison in this paper, since data for similar concentrations of sulphuric acid for FRP and HDPE are not available in literature and it would be logical to assume that the sulphuric acid absorption of FRP will be greater than that of water absorption since Sulphuric acid is a much more aggressive medium than water.
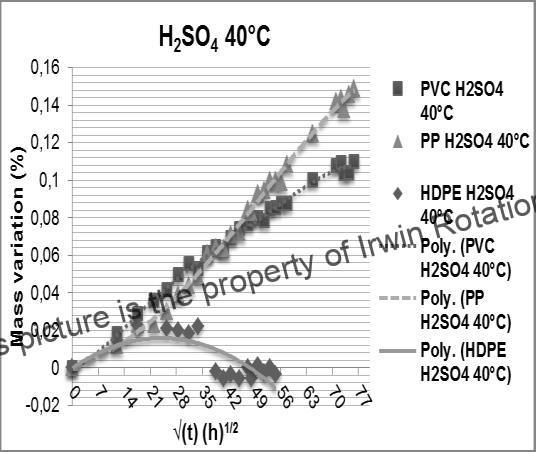
Fig 3. Weight change as a function of time (√t (h1/2)) for HDPE PVC, PP and HDPE exposed to: sulfuric acid solution (H2SO4) at 40°C (7)
Conclusion
While FRP is a superior choice compared to HDPE for applications requiring resistance to Organic solvents, HDPE scores well over Bisphenol Epoxy based FRP resins for acidic media like Sulphuric acid. HDPE does not pose the threat of delamination of the resin layer from the Glass fibre structural layer due to penetration by water and corrosive media like sulphuric acid. Roto-lined PE leverages the low permeability & corrosion resistance of HDPE and the high structural strength of steel and has a clear edge over Bisphenol based Epoxy resin FRP and merits consideration in Ship Scrubber piping and other similar applications.
References
- Andersson, K. Brynolf, S., Lindgren, F., Wilewska-Bien, M. Shipping and the Environment. Springer Nature, Gothenburg, 2016.
- STI Technical Note for Calculating Sulphuric Acid Concentration, STI-TN-20170818-1
- Stefanie Römhild, “Influence of Liquid Diffusion on the Performance of Polymer Materials in Industrial Applications” Licentiate Thesis in Polymer Technology, May 2007, Royal Institute of Technology, Stockholm.
- J. Yao, G. Ziegmann, “Water Absorption Behaviour and Its Influence on Properties of GRP Pipe” Journal of Composite Materials, Vol. 41, No. 8/2007
- Quenos – General Properties – Technical Guide
- Storage & Drying – Introduction – Water Permeation & Relative Humidity – Athlone Extrusions
- Lasfar Sara et al Int. Journal of Engineering Research and Applications ISSN: 2248-9622, Vol. 4, Issue 2(Version 1), February 2014 pp.670-678



